

The pressure of the gas forces a piston downward, doing work. In an automobile engine, the ignition of a gasoline vapor-air mixture produces a gas at a temperature several hundred degrees above room temperature. All heat engines work on a cyclic principle of extracting thermal energy from some source, converting some of this energy to useful work, and rejecting the remaining energy to something at a lower temperature. Gasoline-powered engines, diesel engines, steam turbines, and gas turbines are examples of heat engines. Accordingly, there is no change in the thermal energy of either, no matter how much thermal energy in either one. When two objects at the same temperature are in contact, no heat flows between either. While in transit, the energy is called heat. The exchange of energy stops when both the hand and water come to the same temperature. The water warms and the thermal energy of the water increases. When one puts a warm hand in contact with cold water, the hand cools and the thermal energy in the hand decreases. The sum of all the random kinetic energies of the molecules is called thermal energy, therefore, thermal energy is directly proportional to the Kelvin temperature. On the Kelvin temperature scale, the average kinetic energy is directly proportional to the Kelvin temperature. No molecule has a definite speed or kinetic energy, but a molecule has a definite average kinetic energy that depends on the temperature. In a substance where the molecules may move around, the motion is random.
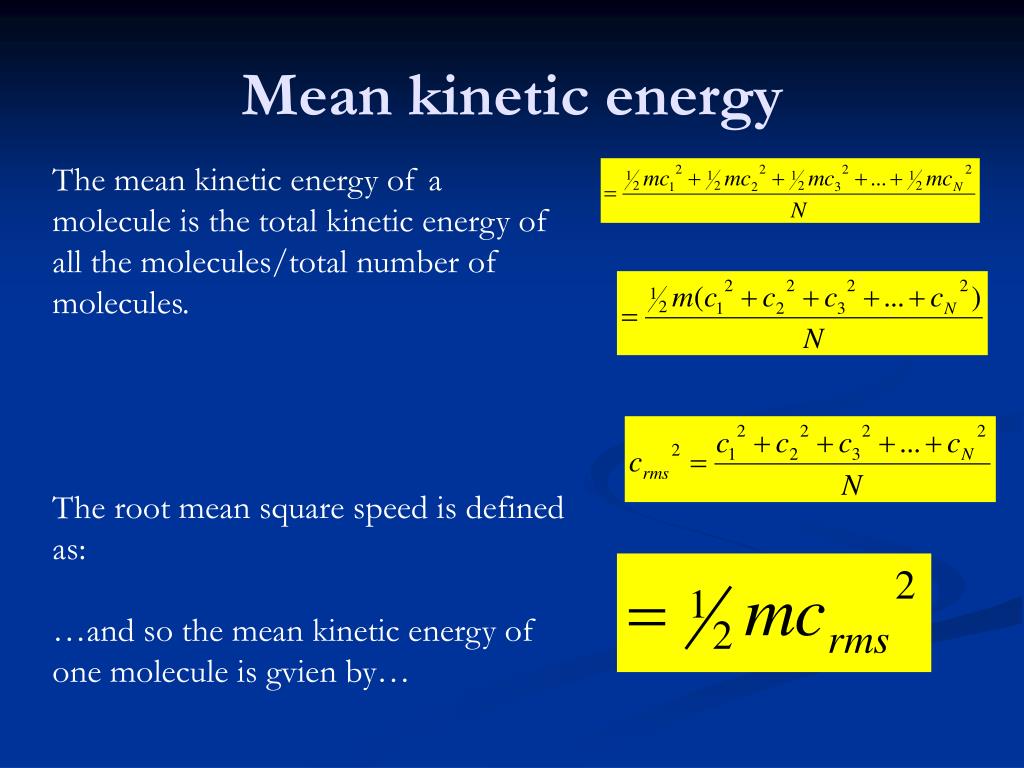
This energy is called internal energy because it is internal to the confines of the substance. Summing the kinetic energy and potential energy of all the molecules gives the total energy of the substance. Depending on the substance, the particles may interact with each other or their surroundings, in which case they would have potential energy along with kinetic energy. Combining mass and speed gives them kinetic energy. Although molecules are microscopic, they do have some mass.
Total kinetic energy of particles free#
In a solid they are not so free to move around, but they can vibrate. In gases and liquids, molecules are relatively free to move around. On the Kelvin temperature scale, thermal energy is directly proportional to temperature.Īll matter is composed of molecules or, in some cases, just atoms. The higher the temperature, the greater the thermal energy. Thermal energy is the sum of all the random kinetic energies of the molecules in a substance, that is, the energy in their motions.


 0 kommentar(er)
0 kommentar(er)
